Engineering Biomedical PCBs for the Future of Healthcare

In modern healthcare, technology isn’t just an assistant—it’s an extension of human capability. Every medical innovation, from life-sustaining implants to AI-powered diagnostic equipment, relies on one silent enabler: the Printed Circuit Board (PCB). At KKPCB, we design and manufacture biomedical PCBs that meet the uncompromising demands of precision, safety, and long-term reliability.
1. The Role of PCBs in Biomedical Systems
Biomedical PCBs form the electronic backbone of medical devices such as pacemakers, defibrillators, imaging systems (MRI, CT, and ultrasound), and increasingly popular wearable healthcare devices. These circuits must perform flawlessly in mission-critical environments where a single electrical fault could endanger a life.
KKPCB’s biomedical PCBs are built with controlled impedance, EMI shielding, and biocompatible materials to ensure stable performance under extreme conditions. Our design philosophy integrates miniaturization, high signal integrity, and thermal stability, aligning with the most stringent global standards such as ISO 13485 and IEC 60601.
2. Advanced PCB Technologies for Biomedical Applications
The future of medical electronics demands smaller, smarter, and more durable circuit systems. To achieve this, KKPCB leverages multiple advanced PCB technologies:
-
HDI (High-Density Interconnect): Enables compact layouts with microvias and fine traces, reducing size while increasing complexity.
-
Rigid-Flex PCB: Combines mechanical durability with design flexibility, ideal for wearable sensors and compact diagnostic tools.
-
Flexible PCB: Made from lightweight polyimide or Teflon materials, allowing integration into irregular or curved enclosures for ergonomic designs.
These technologies collectively support the industry’s shift toward portable and wearable medical solutions, where functionality, weight, and safety must coexist seamlessly.
3. Material Engineering: The Foundation of Performance
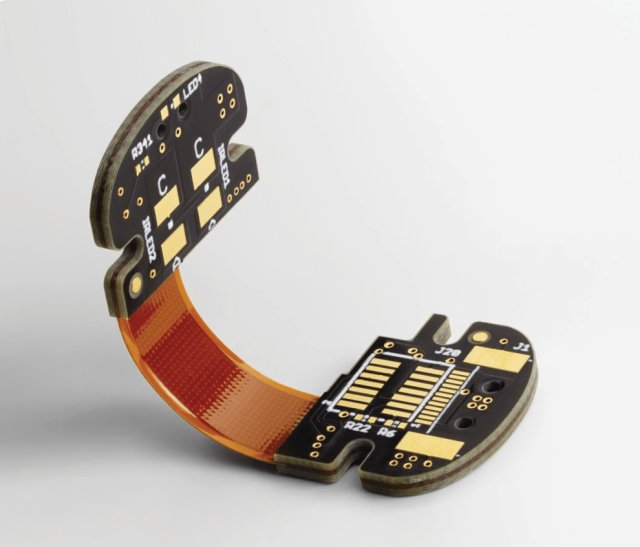
Material selection defines the electrical, thermal, and mechanical behavior of a biomedical PCB.
KKPCB employs a range of advanced substrates depending on application requirements:
-
FR-4 for general-purpose medical electronics.
-
Polyimide for flexible, high-temperature wearable devices.
-
PTFE (Teflon) and Ceramic Laminates for high-frequency or RF medical imaging systems.
Each substrate is chosen to balance dielectric stability, heat dissipation, and mechanical reliability. Our AI-assisted material characterization process ensures optimal substrate pairing with circuit design, improving yield and product lifespan.
4. Reliability Through Design Longevity and Maintainability
In biomedical electronics, long-term reliability isn’t negotiable. Component obsolescence can jeopardize medical certifications, so KKPCB integrates lifecycle forecasting into every project—predicting potential part shortages and pre-qualifying alternatives.
Our PCB layouts prioritize:
-
Optimized thermal paths to avoid hotspots.
-
Strategic test-point placement for easy maintenance and calibration.
-
Controlled height profiles for efficient assembly in sterile manufacturing lines.
By designing for serviceability, KKPCB minimizes downtime and ensures consistent operation throughout the device’s life cycle.
5. Testing, Validation, and Digital Simulation

Every biomedical PCB at KKPCB undergoes a multi-stage validation pipeline, including:
-
Electrical performance testing under variable voltage, temperature, and humidity.
-
Automated optical inspection (AOI) and X-ray layer alignment.
-
Digital Twin simulation, modeling real-world operation to identify weak points before physical prototyping.
This combination of predictive simulation and precision testing guarantees stability in both life-critical implants and high-frequency imaging devices.
6. Compliance and Safety Assurance
Medical-grade PCBs must comply with rigorous global standards.
KKPCB ensures all biomedical products meet or exceed:
-
ISO 13485 (Medical Device Quality Management)
-
RoHS and REACH environmental standards
-
UL94-V0 flame retardancy classifications
Our in-house traceability systems track every process from material sourcing to final inspection, ensuring total transparency and compliance with global healthcare regulations.
KKPCB: Engineering the Next Generation of Biomedical Intelligence
Innovation in medicine depends on precision in electronics. KKPCB combines decades of PCB engineering expertise with advanced manufacturing technology to deliver circuits that power the future of healthcare.
From implantable sensors to diagnostic imaging modules, our biomedical PCB solutions redefine reliability and performance at every scale.
When precision saves lives, KKPCB is your trusted partner in building the next generation of biomedical systems.

